The Periodic Table's 4 New Elements
In June 2016, it was publicly announced that the periodic table will have four new elements added to it. The International Union of Pure and Applied Chemistry (IUPAC) has worked with those laboratories that discovered the elements to name them and has now released the new periodic table for a five-month public review period which expires on the 8th of November, 2016. After the review period, the IUPAC Council will formally approve the revised periodic table.
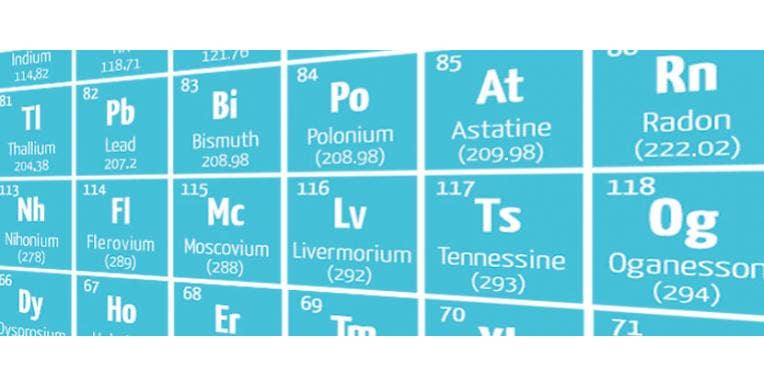
The new elements will be added to the lower right-hand corner and consist of:
- Nihonium (symbol Nh) – atomic number 113
- Moscovium (symbol Mc) – atomic number 115
- Tennessine (symbol Ts) – atomic number 117
- Oganesson (symbol Og) – atomic number 118
In January, the new elements were first announced and given interim names until formal names were released. The rights to name the elements were given to those research teams that discovered them. The US, Russia, and Japan were credited with the discovery of the new elements and the names were announced in June. Whilst these teams have the rights to name the elements, they do have to follow a naming convention as outlined by the IUPAC which states that any new element must be named after either:
- a mythological concept or character (including an astronomical object),
- a mineral or similar substance,
- a place, or geographical region,
- a property of the element, or
- a scientist.
The Meaning Behind the Names
- Nihonium – Nihon is one way to say “Japan” in Japanese. As this was the first element discovered by an Asian country, Japan wanted the name to represent the geographical region. Nihon means “the Land of Rising Sun” and provides a direct connection to Japan as a nation.
- Moscovium – Moscovium also represents a geographical region, specifically, the Moscow region which is where the discovery experiments were conducted.
- Tennessine – Tennessine recognises the laboratories that contribute to element research in the Tennessee region which include Oak Ridge National Laboratory, Vanderbilt University, and the University of Tennessee in Knoxville.
- Oganesson – Oganesson attributed its name to a scientist following the IUPAC naming convention. Professor Yuri Oganessian (born 1933) is a Russian nuclear physicist who is credited with three confirmed element discoveries.
How Exactly Were These Elements Found?
Firstly, of the 118 known elements, 94 were found naturally on Earth. This leaves the remaining 24 elements that are not found naturally, meaning they were created in a lab. Creating “man-made” elements involves using particle accelerators to smash atoms together in the hope that they may fuse to become a new element. If the proton count is one that has never been reached before, a new element is formed. To become a new element, however, the experiment must be recreated or reproduced by other scientists to ensure they get the same result.
Post November and provided all goes well with the public review period, the periodic table will be officially updated to include the four new elements. Westlab are getting in early and releasing updated wall hanging periodic tables within the next couple of months. If, for whatever reason, the updated periodic table is rejected during the public review period, Westlab will refund in full the purchase price of the periodic table!
UPDATE JANUARY 2017: As of December 2016, the 4 new elements were approved and formally added to the periodic table.