How to Read a Meniscus in Chemistry
Liquids are difficult to measure with the naked eye due to surface tensions causing a meniscus to form on the circumference of the measuring object. In this article, you will learn what a meniscus is, why it forms, and how you can accurately and precisely measure the volume of a liquid in a laboratory graduated measuring glassware or plasticware.
What is Meniscus Curve?
The meniscus curve of a liquid is the upward or downward curve seen at the top of a liquid in a container. The nature of the curve whether upward (convex) or downward (concave) depends on the surface tension the liquid and its adhesion capacity to the wall of the container.
Why does a Meniscus form?
The generation of a meniscus phenomenon can be attributed to adhesion and is partially influenced by the relatively high surface tension of the fluid. The molecules of the fluid exhibit an affinity towards the molecules present in the wall of the glass beaker. As the fluid molecules have an inherent tendency to cohere, they attract and adhere to their neighboring molecules, thereby establishing a bond with the molecules on the glass surface. The cumulative effect of these interactions results in the formation of the meniscus.
Concave vs. Convex Meniscus: What is their Difference?
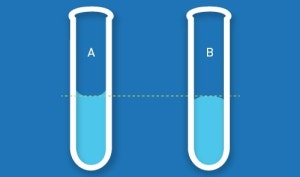

1. Concave Meniscus (See diagram A)
A concave meniscus occurs when the molecules of the liquid are strongly attracted to the container wall rather than to each other. In that case, the liquid appears to ‘stick’ from the edges forming a concave shape.
2. Convex Meniscus (See diagram B)
A convex meniscus appears when molecules of a liquid are strongly attracted to each other rather than to the wall of the container. Most liquids, including water, show a concave meniscus but a great example of a convex meniscus is liquid mercury in a glass container. It is to be noted that in some cases, the meniscus appears in a straight line instead of any curvature at all. For example, water in some plastic cylinders. No meniscus makes measuring very easy.
Read more, Click here to know about blood plasma and serum.
How to read a meniscus correctly
You must get at eye level with the meniscus to obtain an accurate reading. Pick up the glassware to bring it up to eye level or bend down to take a measurement.
The key to getting an accurate reading is to measure the center of the meniscus whether it be concave or convex. E.g. with a concave meniscus, measure the bottom of the meniscus and for a convex meniscus, take the reading from the top of the meniscus. (See diagram reading line in diagrams A and B).
How do you read the Meniscus of Water?
In order to obtain an accurate measurement of the meniscus of water, it is imperative to align the reading line with the center of the curved surface. Fr water, the lowest point of the meniscus corresponds to the center. It is crucial to ensure that the measurement is being taken from the midpoint of the meniscus for a correct reading..
FAQs
Q. What is a curved surface in a graduated cylinder called?
The liquid’s curved surface in a graduated cylinder is called the meniscus.
Q. Do all liquids have a meniscus?
Almost all liquids have a meniscus but water has the highest surface tension, making its meniscus levels more prominent and suitable for classroom learning.
Q. What part of the meniscus curve determines your measurement?
The bottom of a concave meniscus or the top of a convex meniscus are the parts of a meniscus curve that determine your measurement.